Water and computers don’t mix well in reality as everyone knows. Modelling the influence of water on biological and chemical processes also poses a whole set of challenges. However, it enables me to investigate a very interesting question: What do molecules look like in future oceans and what are their characteristics?
Why are molecules in future oceans of interest?
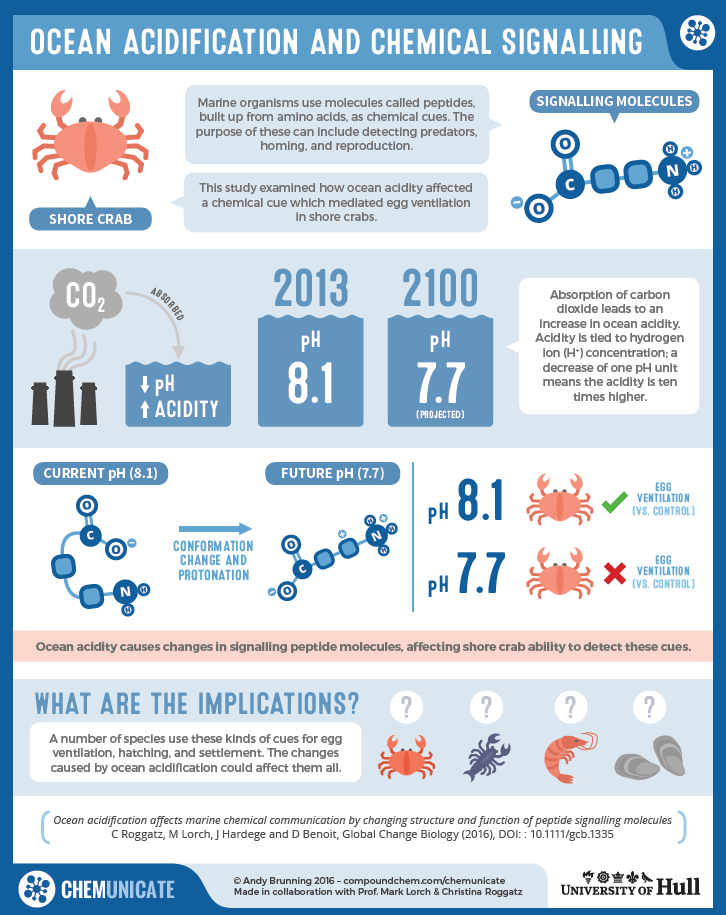
The oceans are becoming ever more acidic as man-made, increasing carbon dioxide emissions into the atmosphere are absorbed and cause the pH in our world’s oceans to decrease an effect called ocean acidification. By the year 2100, the sea surface pH is predicted to drop by up to 0.4 units to pH 7.7. This may not seem much, but it has been shown to significantly affect the fitness, physiology, reproduction and behaviour of everything in the sea from huge sharks and whales to the tiniest of plankton. My latest research also reveals that smell molecules in the ocean are significantly affected by ocean acidification (Fig. 1).
Imagine you are a little crab living on a shore covered with large rocks and deep pools, and battered by tides and waves. The only way to find your lunchtime snack would be to smell it from a distance. But the same also applies to the octopus hunting you. So you, the crab, also rely on smelling the octopus first to avoid being eaten. What if this was no longer possible?
In fact, chemical communication using smell is essential for marine organisms. Its importance is comparable to the combined status of vision and hearing in humans. Smell molecules are chemicals that are produced on purpose – by females to attract males, for instance – or as a result of natural processes such as protein degradation. In both cases, they can be used by animals to smell their way around. Many smell molecules transporting information from its source to an organism are potentially sensitive to pH. This holds particularly true for peptides and proteins, which are used, for example, by crabs, barnacles and mussels as markers for food, predators, settlement spaces or during brood care and larval hatching. The main question is whether a drop in pH will affect their function and render them ineffective.
How can Viper be used to model marine molecules?
For a molecule to be “smelled” by an animal it needs to interact with a receptor located in the animal’s nose – although crabs actually smell using their antennae and even the tips of their legs. Two characteristics are key in this process: the molecule’s conformation (3D structure) and how positive and negative charges are distributed. Both turn out to be affected by changes in pH.
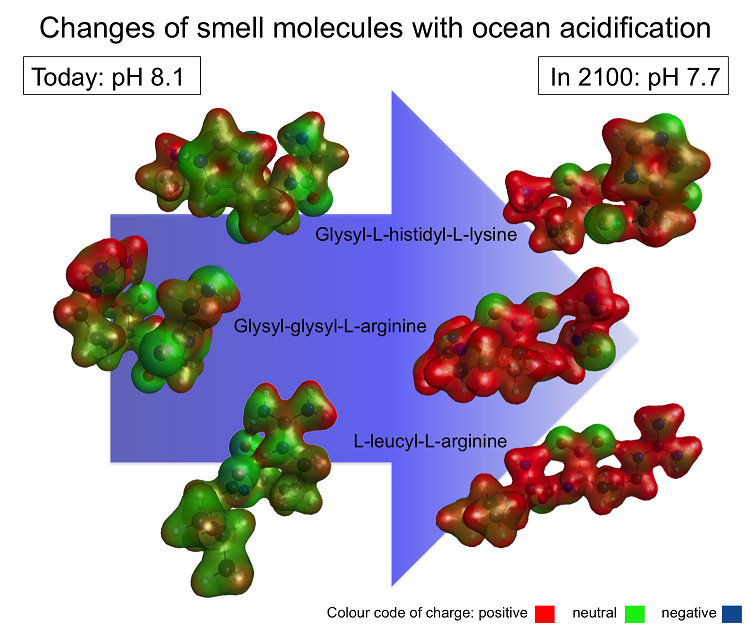
Most smell molecules are too small to gain insight into their conformation using chemical analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy. Also the different pH dependent forms often cannot be studied separately. I therefore use Viper to assess what smell molecules in future ocean conditions will look like. Using quantum chemical programs, Viper allows me to calculate the most likely conformation of a molecule. This can be achieved by altering the position of the atoms in the molecule to obtain the arrangement that has the lowest possible energy and so the optimal conformation is found. Viper also enables me to visualise the charge distribution around the molecule. The differences between molecules in today’s and future ocean conditions can so be shown by comparison, for example, for three smell molecules used by crab larvae to communicate (Fig. 2). While in their eggs, tucked underneath the female’s abdomen, the larvae release these smells to tell their mother when they need more ventilation, oxygen or assistance to hatch. Molecules responsible for the smell today are relatively compact and have very distinct areas of positive and negative charge. In contrast, the future molecules are less compact and have an overall positive charge. These changes were found to happen exactly within the ocean pH range expected from today until the year 2100.
Computational models are a great way of visualising differences. However, the results are only of significance if the model conformers can be verified by experimental data. I gathered experimental data by measuring the NMR spectra for samples with a known concentration of the molecule in a buffer solution. The pH of each sample can be adjusted to a value of interest using acid or base and the buffer solution helps to stabilise the pH during the measurements and to mimic ocean conditions in a test tube to a certain extent. Verifying the computational model conformers is then possible by calculating the NMR parameters of each model conformer and comparing them to the experimental data of the molecule. During my PhD, I have been working on the development of a suitable validation method and a way to obtain the best possible model conformers. I therefore investigated in detail the effects of calculation method, molecular conformation and representation of water on the calculation of NMR parameters. Using Viper to run jobs with 14 cores in parallel reduced the computation time from more than one day to only half an hour. The detailed insights gained from those calculations are currently analysed and will soon be published in a series of papers on the effects of different factors on NMR calculations of small biomolecules.
For more information on Christina Roggatz’s work, please see:
- The research paper Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules
- The Conversation article Oceans may become too acidic for animals to smell their way around
- Email roggatz@outlook.com
If you wish to contribute a case study based on your HPC work, please contact: viper@hull.ac.uk


